The intestine is a fascinating and complex environment! As only a single layer of epithelium separates “you” from the outside world, here intestinal epithelial cells, immune cells, bacteria, and other luminal contents work together in close proximity to maintain gut homeostasis. Perturbing any of these populations, for example though altered epithelial cell function, aberrant immune activation, or microbiome dysbiosis, can have wide-reaching deleterious effects. Our research aims to further our understanding of these normal processes and how altering cellular function can contribute to intestinal disease and progression. Keep reading for more specifics on our research and ongoing lab projects!
Research
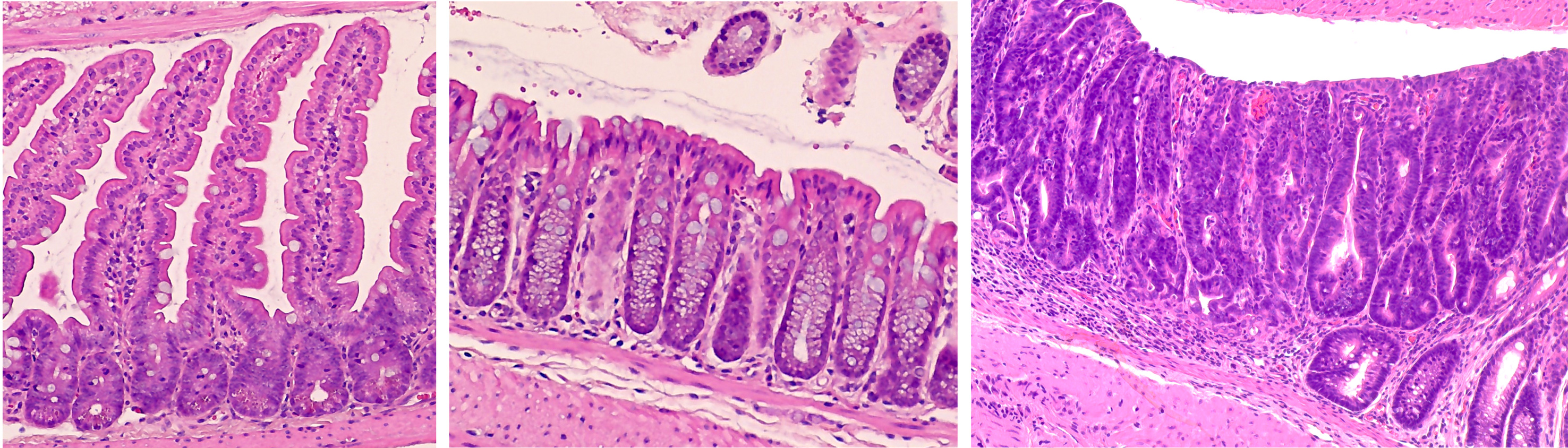
Focus #1: How does ROS, and the proteins which regulate it, contribute to intestinal homeostasis and disease?
Reactive oxygen species, or ROS, are a collection of highly reactive oxygen-containing molecules. In cancer, high levels of ROS are believed to promote tumor initiation by increasing DNA mutation, inflammation, and activity of growth-related signaling pathways. However, non-pathogenic levels of ROS are produced as part of many cellular processes and help to regulate multiple aspects of the intestinal microenvironment at homeostasis.
ROS is regulated, in part, by endogenous antioxidant proteins. Our work focuses on one family of these proteins, the glutathione peroxidases. These proteins can incorporate elemental selenium into their structure and are believed to mediate much of selenium’s known antioxidant effect. So far, our lab has discovered a role for glutathione peroxidase 1 (GPx1) in regulating intestinal proliferation, inflammation and colitis severity, microbiome composition, early colon tumor development, and response to chemotherapy. Future studies will work to tease out the mechanisms which drive these phenotypes and expand our work into other members of the glutathione peroxidase family.
|
|
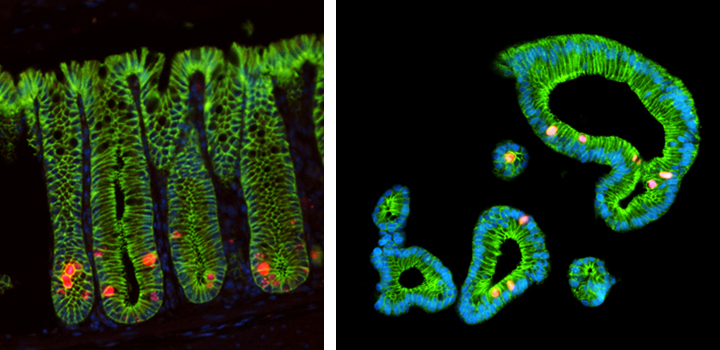
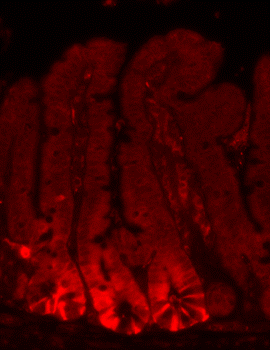
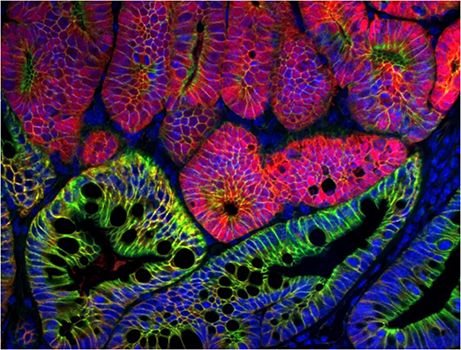
Focus # 2: How are intestinal stem cell programs altered in colon cancer development, progression, and therapy?
The epithelium in your gut undergoes constant renewal. You may not realize, but you have an almost entirely new gut epithelium approximately every 7 days! This massive amount of proliferation is mostly maintained by a specific population of adult intestinal stem cells (ISCs) that reside in the crypt base. However, as is often observed in cancers, the aberrant activation of cellular programs necessary for ISC function are potent inducers of intestinal tumorigenesis. Our research aims to uncover more pathways important for ISC biology that are 1) deregulated in tumorigenesis and 2) can be therapeutically targeted in the context of colon cancer.
|
|
|
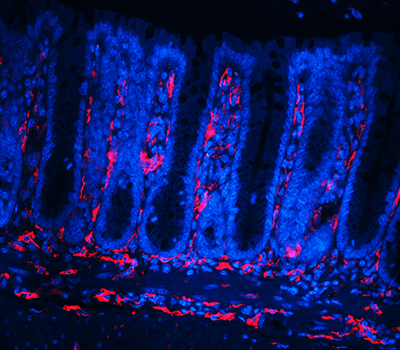
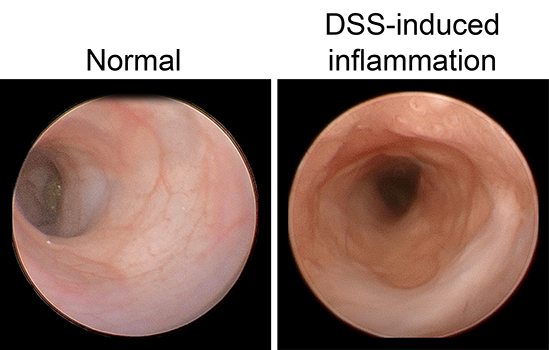
Although ISCs are crucial for maintaining intestinal proliferation and healing the intestine after injury, actively cycling ISCs are also exquisitely sensitive to a number of biological insults. Interestingly, these insults include many types of therapies given to cancer patients, from radiation, to chemotherapies, to checkpoint inhibitor treatment. This stem cell death can drive GI symptoms and colitis that are common side effects in cancer therapy, which greatly decrease quality of life and can lead a patient to discontinue therapy. Ultimately, we hope that these studies can help identify ways to maintain proper gut function in the setting of intestinal injury that can benefit patients with inflammatory bowel disease (IBD) or colitis induced by cancer therapy.
|
|
|
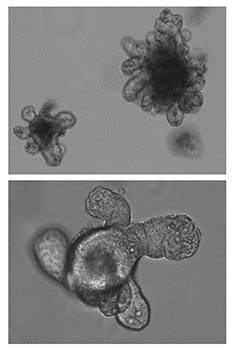
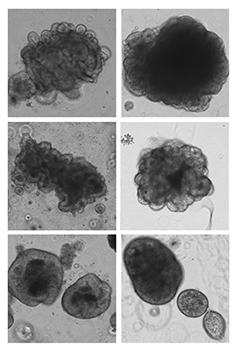
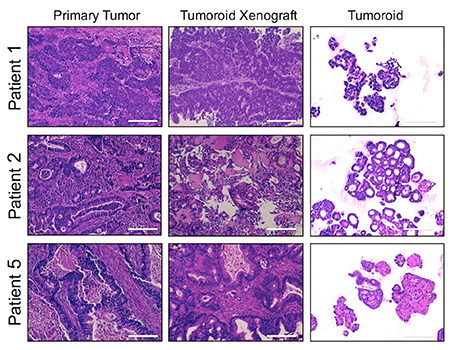
Focus #3: How can we better model tumor growth and therapeutic responses with intestinal organoids?
The intestine is a complex organ. Unfortunately, many of these complexities are lost in standard 2D cell cultures. Over the past 15 years, methodology has been described to establish 3 dimensional cultures from the gut epithelium which can be maintained indefinitely. When made from normal intestine, these cultures maintain many of the differentiated cell types and structure observed in vivo. When made from tumor tissues, these cultures likewise recapitulate much of the heterogeneity and variability seen in the patient tumor. In our lab, we feel that good models make better science! While we often use patient-derived organoids as a faithful system in which to study particular genes and pathways, future research will aim to use organoids as a non-biased method to identify novel cancer-relevant proteins and model patient-specific therapeutic responses.
|
|
|